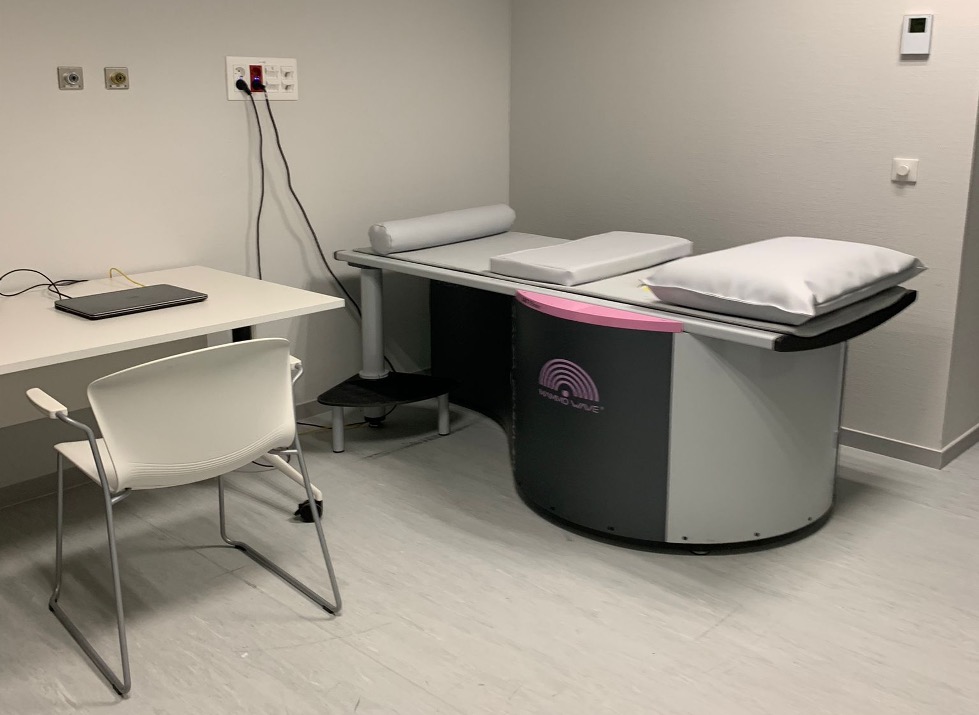
SESCAM (Servicio de Salud de Castilla-La Mancha) is the public health service responsible for managing healthcare across Castilla-La Mancha, Spain. Established in 2002, SESCAM provides healthcare services to over two million people, encompassing primary care, specialized care, emergency services, and public health programs across a network of health centres, hospitals and clinics.
SESCAM’s mission is to deliver comprehensive, high-quality, and universal healthcare, free at the point of access. The organization prioritizes preventive healthcare programs — such as vaccination campaigns, cancer screening, and health education—to enhance public health outcomes in Castilla-La Mancha. With a commitment to improving quality and accessibility, SESCAM also emphasizes digital services for patients, aiming to bridge healthcare gaps in rural areas and meet rising demands in a growing population.
The SESCAM team involved in the MammoScreen project is based in the Radiology Department at the University Hospital of Toledo. Led by Dr. Cristina Romero Castellano, an expert in the use of AI in mammography, the team is based on a research group – Group in Clinical Research Applied to Diagnostic Imaging (Grupo de Investigación Clínica Aplicada al Diagnóstico por Imagen, ICADI), that includes experienced breast radiologists, biomedical engineers, and a dedicated project manager. Currently, the Breast Radiology Unit at SESCAM oversees the Regional Early Detection Program for Breast Cancer, serving approximately 85,000 women in the Toledo healthcare region.
The clinical team is committed to continually optimizing protocols and incorporating new devices and methodologies to improve diagnostic accuracy and efficiency. This commitment to innovation has earned SESCAM recognition from the European Commission, including an award for best practices in breast cancer diagnosis and management (Optimized Population-Based Screening and Diagnostic Pathway for Breast Cancer Detection, Best Practices in NCD Prevention 2021).

Role of SESCAM in the MammoScreen Consortium
Within the MammoScreen Consortium, SESCAM is responsible for developing the clinical trial protocol and related documentation. SESCAM ensures a cohesive approach across all participating recruitment centres, working closely with the UBT team and the CRO to create an electronic case report form (e-CRF) that simplifies data collection. The dedicated clinical trial protocol has been recently published in BMJ Open. As the project’s clinical coordinator, SESCAM oversees clinical ethics and recruitment strategies and ensures that standard screening data from mammograms and other radiological or histological sources are accurately captured in the e-CRF. This standardization aligns the trial’s outcomes with European guidelines on breast cancer screening and diagnosis.
As this is a novel technology, there are currently no established guidelines or recommendations for its use in breast lesion or breast cancer detection. The absence of official endorsements from scientific societies at the national, European, and international levels reflects the need for a stronger base of scientific evidence. For instance, through the MammoScreen project, SESCAM and its Consortium Partners are working together to build this crucial evidence base, aiming to drive a paradigm shift in breast cancer screening and diagnosis.
Collaborative Efforts and Community Engagement
SESCAM plays a vital role in engaging with a diverse group of stakeholders, including clinical and regulatory authorities and end users—primarily women in the general population who are invited for breast screening. To support these activities, SESCAM collaborates closely with the ELAROS team in developing the clinical web portal and user-friendly mobile app, which are key outcomes of the MammoScreen Project. Additionally, SESCAM is instrumental in organizing workshops with clinicians to promote knowledge sharing and esure alignment with the project’s goals.
Advancing Breast Healthcare Through Innovation
SESCAM’s contribution to the MammoScreen Project is essential for integrating MammoWave into established breast imaging standards, paving the way for this innovative screening technology to become a part of routine breast healthcare. Stay tuned to the MammoScreen communication channels as well to learn more about their activities in the project.
You can keep up with SESCAM´s team activities by following their social networks: Instagram, X, Facebook, and LinkedIn.